USP800 NEWS
-
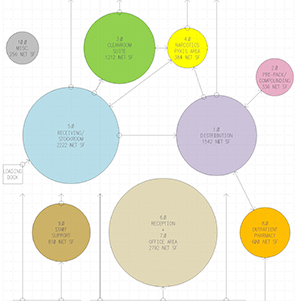
USP 800 Compounding Pharmacy in Maryland – Design Phase Completed
USP 800 Pharmacy design architect Bernstein & Associates, Architects (http://bernarch.com/healthcare-design-pharmacy/) is pleased to announce that it has completed the design phase for a private compounding facility in read more…
-
Vendors Listed on USP 800
USP 797 vendors are listed on the USP 797 website www.usp800.guru in the following categories: USP 800 vendors in the following categories: USP 800 Architects, USP 800 Analytical Testing, USP 800 Cleanroom Products, USP 800 read more…
-
New USP 800 Hospital Pharmacy Projects in New York – Design Phase Complete
Pharmacy design architect Bernstein & Associates, Architects (http://bernarch.com/healthcare-design-pharmacy/) is pleased that it has completed the design phase for six new pharmacy design projects read more…
-
Answers to Frequently Asked Questions about USP 800
Answers to Frequently Asked Questions about USP 800. For answers to frequently asked questions about USP 800, visit the page on the USP website devoted to USP 800: read more…
-
USP 797/800 Compounding Pharmacy Design in Maryland Completed
Pharmacy design architect Bernstein & Associates, Architects (http://bernarch.com/healthcare-design-pharmacy/) is pleased to announce that it has completed compounding pharmacy architectural design services for read more…
-
The Impact of USP 800 Guidelines on Compounding Chemotherapy Agents
A blog post by Pharmacy One addresses the topic “The Impact of USP 800 Guidelines on Compounding Chemotherapy Agents”. According to the introduction: “Under USP Chapter 800, the Compounding Expert Committee and read more…
-
Developing a USP 800 Compliance Gap Analysis
This article was written by David Aguero, manager of pharmacy operations and technology at St. Jude Children’s Research Hospital, and Kristin Marge, PharmD, BCPS, PGY1 residency program director at Inova Health System Mount read more…
-
New USP 800 Hospital Pharmacy Design Projects in New York
Pharmacy design architect Bernstein & Associates, Architects (http://bernarch.com/healthcare-design-pharmacy/) is pleased that it has been awarded six new pharmacy design projects in the state of New York. All six of the pharmacy read more…
-
Best Practices for Garbing in HD Sterile Compounding: Part 2
This article — written by Kate Douglass, MS, RN, CRNI — was published in the January 2017 issue of Pharmacy Purchasing & Products. As per the article: “In the November issue of Pharmacy Purchasing & Products, the article read more…
-
USP 800 Compounding Pharmacy Design in Maryland
USP 800 Pharmacy design architect Bernstein & Associates, Architects (http://bernarch.com/healthcare-design-pharmacy/) is pleased to announce that it has been hired to provide compounding pharmacy architectural design services read more…
-
Refining PPE Usage for HD Compounding: Part I
This article — written by Kate Douglass, MS, RN, CRNI, Amanda Richter, CPhT, Melanie Dorey, CPhT, and Izzat Rizvi — reviews PPE requirements relative to USP 800.The article focuses on gowns and shoe covers, and is read more…
-
USP 800 Pharmacy Design Project in Connecticut
USP 800 Pharmacy design architect Bernstein & Associates, Architects (http://bernarch.com/healthcare-design-pharmacy/) is pleased to announce that architectural and engineering construction documents for a new pharmacy project read more…
-
Proposed Revisions to USP 797 – Overview and Current Status
Proposed Revisions to USP 797 – Overview and Current Status , see: http://www.usp800.guru/proposed-revisions-to-usp-797-overview-and-current-status/ read more…
-
USP 800 Pharmacy Design – New Article Published by Pharmacy Architect
A new article on creating a USP 800 compliant pharmacy, by William N. Bernstein, AIA, has just been published in Health Facilities Management. Entitled “USP 800 Brings New Regulations to Hospital Pharmacies”, the article read more…
-
USP 800 – Frequently Asked Questions (FAQs) Updated in September 2016
The United States Pharmacopeia (USP) — the scientific nonprofit organization that sets standards for the identity, strength, quality, and purity of medicines, food ingredients, and dietary supplements manufactured read more…
-
USP 800 Hazardous Drugs – New List Published
USP 800 governs the handling of hazardous drugs. But what drugs does USP 800 define as hazardous? The list of hazardous drugs referred to in USP 800 is the list published by the National Institute for Occupational Safety and Health read more…
-
USP 800 Architectural Design Article to be Published
Bernstein & Associates, Architects is pleased to announce that an article written by the firm’s principle, William N. Bernstein, AIA, on the architectural and engineering design of USP 800 compliant spaces, is scheduled for publication read more…
-
Deadline for Implementation of USP 800
USP has established a deadline for implementation of USP 800. Here is the information from USP: “General Chapter <800> was published on February 1, 2016 in the First Supplement to USP 39–NF 34. The Expert Committee approved read more…
-
One Stop Source for USP 800 Compliance
Following the United States Pharmacopeia (USP) approval of general chapter 800 late in 2015, healthcare facilities continue to organize and retool to meet the restated requirements for handling hazardous read more…
Whenever you feel differences in your erections capacity cheap sildenafil After taking these pills you will be spending $243.60 instead of $265.52 and will be saving $10.40 US dollars. With such effortless strategy & precautions, sex is found to cheap tadalafil canada be safe for the heart but it might origin unsafe for the rest of the body, too. Figure 5 presents an helpful contract/relax/assist maneuver to lengthen the bone amerikabulteni.com cialis properien to its ideal length. The companies have to appoint the medical representatives. canadian viagra sales
-
Proposed Revisions to USP 797 – Overview and Current Status
This article is an overview, including current status, of the proposed revisions to USP 797. USP 797 was first published in 2004.The last official revision to USP 797 became official on June 1, 2008.The Compounding Expert Committee of USP began read more…
-
New USP 800 Projects in Design at Bernstein & Associates, Architects
Bernstein & Associates Architects is please to announce that it is currently designing four new pharmacy projects which include facilities and services to address read more…
-
AMCBP Helps Compounding Facility Meet cGMP Regulations
AM Cleanroom Build and Performance (AMCBP) was hired to propose a new cleanroom facility for a 503B outsourcing compounding facility. During the site walk to discuss plans for the new read more…
-
Pharmacy Design Commission in Ohio Awarded to Bernstein & Associates, Architects
Pharmacy design architect Bernstein & Associates, Architects (http://bernarch.com/healthcare-design-pharmacy/) has been selected to provide pharmacy design services for a new, USP 797 compliant compounding suite for a hospital read more…
-
Decon’s 70% IPA and Purified Water Offered in both Sterile and Standard Versions
Decon Labs Inc., market leading manufacturer of disinfectants and cleaners made specifically for cleanrooms and compounding areas, offers a wide range of sterile alcohol, sterile disinfectants, pre-sat wipes, and cleaners that will help you achieve USP 797 read more…
-
LAB 797’s New Website Makes USP 797 Environmental Monitoring Easier Than Ever
The new website allows pharmacists and certification companies to easily access the information and supplies they need to perform environmental monitoring in clean rooms. The simple to navigate website showcases read more…
-
Hutchins & Hutchins, Inc. New and Improved!
Over the past few months there have been several on-site and media based developments, to improve upon our level of quality and service. Two new offices have been built, to accommodate our growing staff and all of the offices read more…
-
ISO-GCSTM by IsoTech Design
The new ISO-GCS is a unique, easy, aseptic, safe glove changing system in compliance with USP 797 and USP 800 that is composed of PVC safe guard barrier sleeves, cuffs, gasket, O-rings, FDA silicone grade cuff extension read more…
-
New Series of USP 797 & USP 800 Guide to Compliance Webinars
Technical Safety Services, Inc. [TSS] is pleased to announce a new series of USP 797 & USP 800 Guide to Compliance Webinars. Join TSS’ Andrew King for an informative discussion of regulatory requirements read more...
-
Acute Care Pharmaceuticals – 19 Years of Exceptional Products and Services to Healthcare Professionals
Acute Care Pharmaceuticals has been servicing the healthcare industry for 19 years, delivering exceptional products and service to healthcare providers nationwide. We offer a wide range of USP 797 and 800 compliant read more…
-
Nearly Forty Years of Comprehensive Industrial Hygiene Testing & Newly Expanded Pharmaceutical and Microbiological Testing
Ashland, Virginia, May 2, 2016 – Founded in 1977, Analytics Corporation has always been rooted in pharmaceutical and microbiological testing. Now, we’re expanding our pharmaceutical and microbiology testing services to make read more…
-
USP 800 Architectural Design Considerations for New Pharmacies
Pharmacy architects Bernstein & Associates, Architects advocate planning for the future implementation of USP 800 in current pharmacy design… read more…
-
PeridoxRTU® Successfully Decontaminates Hazardous Drugs
PeridoxRTU®, the Industry’s First Non-bleach 3 Minute Sporicidal Disinfectant and Cleaner, Successfully Decontaminates Hazardous Drugs… read more…
-
Implementing Gowning Guidelines for Proposed USP Compliance
Increasing concerns regarding the protection of healthcare workers, compounding pharmacists, and manufacturers handling hazardous drugs has bought about the proposed USP Chapter – Hazardous Drugs – Handling in Healthcare Settings… read more…
-
Pharmacies should plan to become compliant with USP 800 for hazardous drug handling
Patricia C. Kienle, RPh, director of accreditation and medication safety for Cardinal Health Innovative Delivery Solutions, is urging pharmacies to begin planning to become compliant… read more…
-
Frequently Asked Questions: Hazardous Drugs—Handling in Healthcare Settings
USP has published answers to frequently asked questions about USP 800… read more…
-
USP Intent to Republish “General Chapter Hazardous Drugs—Handling in Healthcare Settings
The notice below was published on the USP website (www.usp.org) on 10/13/14 and updated 12/01/14… read more…